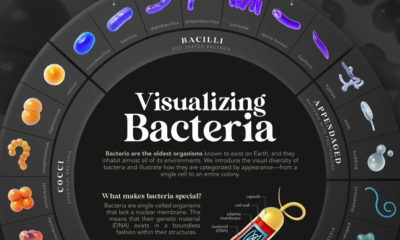
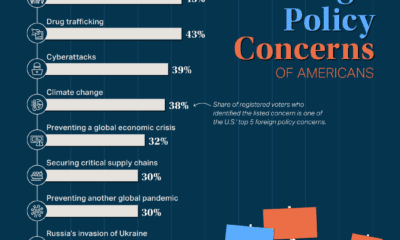
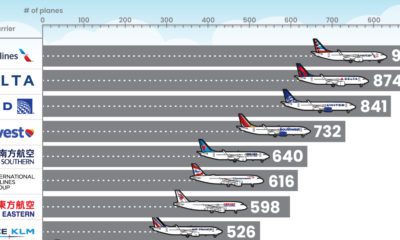
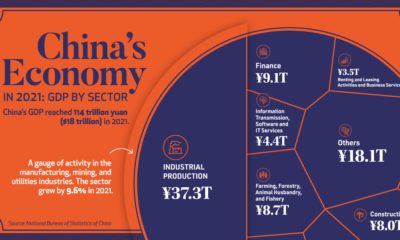
Soon after it received the label “Delta,” it started to become the predominant strain of COVID-19 in countries of transmission. It spread faster than both the original disease and other variants, including “Alpha” that had taken hold in the UK. Now the COVID-19 pandemic has essentially become the Delta pandemic, as the variant accounts for more than 90% of global cases. But how does the COVID-19 Delta variant differ from the original disease? We consolidated studies as of September 2021 to highlight key differences between COVID-19 and the dominant variant. Sources include the CDC, Yale Medicine, and the University of California.
COVID-19 vs COVID-19 Delta Variant
At first glance, infections caused by the Delta variant are similar to the original COVID-19 disease. Symptoms reported from patients include cough, fever, headache, and a loss of smell. But studies showed that the difference was in how quickly and severely patients got sick:
Spread rate: How quickly the infection spreads in a community (based on the R0 or basic reproductive number). The Delta variant spread 125% faster than the original disease, making it potentially as infectious as chickenpox. Viral load: How much of a virus is detectable in an infected person’s blood, with higher loads correlating with more severe infections. Delta infections had a 1000x higher viral load. Virus detectable: How long after exposure a virus is detectable in an infected person’s blood. Delta infections were found to be detectable four days after exposure, faster than the original disease (six days). Infectious period: How long an infected person has the capability to pass on the virus to other people, from the first time they were exposed. Delta infections were contagious for longer than traditional COVID-19 infections, at 18 days compared to 13 days. Risk of hospitalization: How much more or less likely is an infection going to require hospitalization for treatment? Infections caused by the Delta variant were twice as likely to cause hospitalization compared to the original disease.
One other important finding from studies was that the existing COVID-19 vaccines helped against Delta infections. The CDC found that approved vaccines reduced the rate of infection by 5x and the rate of hospitalization by 29x in a breakthrough case. They also found that overall efficacy against infection can wane over time, however, and at-risk people might require a booster vaccine.
What About Other COVID-19 Variants?
Delta is just one of many COVID-19 variants tracked by health officials, but it’s the one we know the most about. That’s because reliable statistics and information on diseases requires thousands of cases for comparisons. We know a lot about Delta (and the once-dominant UK strain Alpha) because of how widespread they became, but there haven’t been enough cases of other variants to reliably assess differences. As of September 2021, WHO was tracking 20 COVID-19 variants around the world with different classifications based on potential severity:
14 Variants under monitoring (VUM): Variants that are deemed to not pose a major global health risk, or no longer pose one. 2 Variants of Interest (VOI): Variants that affect transmissibility, virulence, mutation, and other virus characteristics, and are spreading in clusters. 4 Variants of Concern (VOC): Have similar characteristics to VOI but are further associated with a global risk.
Most of the current variants of interest and concern were first identified and labeled in late 2020, though 2021 variants are showing up as well. Should you be worried about all of these variants? For the most part, a lack of cases to provide clear information also reflects that they’re equivalent to or weaker than traditional COVID-19 infections. But it’s important to note that our understanding of diseases and variants becomes more nuanced and accurate over time. As research continues over a longer timeline and over a wider database of cases, expect information on COVID-19 variants (and any disease) to become more concrete. on The leading options for preventing infection include social distancing, mask-wearing, and vaccination. They are still recommended during the upsurge of the coronavirus’s latest mutation, the Omicron variant. But in December 2021, The United States Food and Drug Administration (USDA) granted Emergency Use Authorization to two experimental pills for the treatment of new COVID-19 cases. These medications, one made by Pfizer and the other by Merck & Co., hope to contribute to the fight against the coronavirus and its variants. Alongside vaccinations, they may help to curb extreme cases of COVID-19 by reducing the need for hospitalization. Despite tackling the same disease, vaccines and pills work differently:
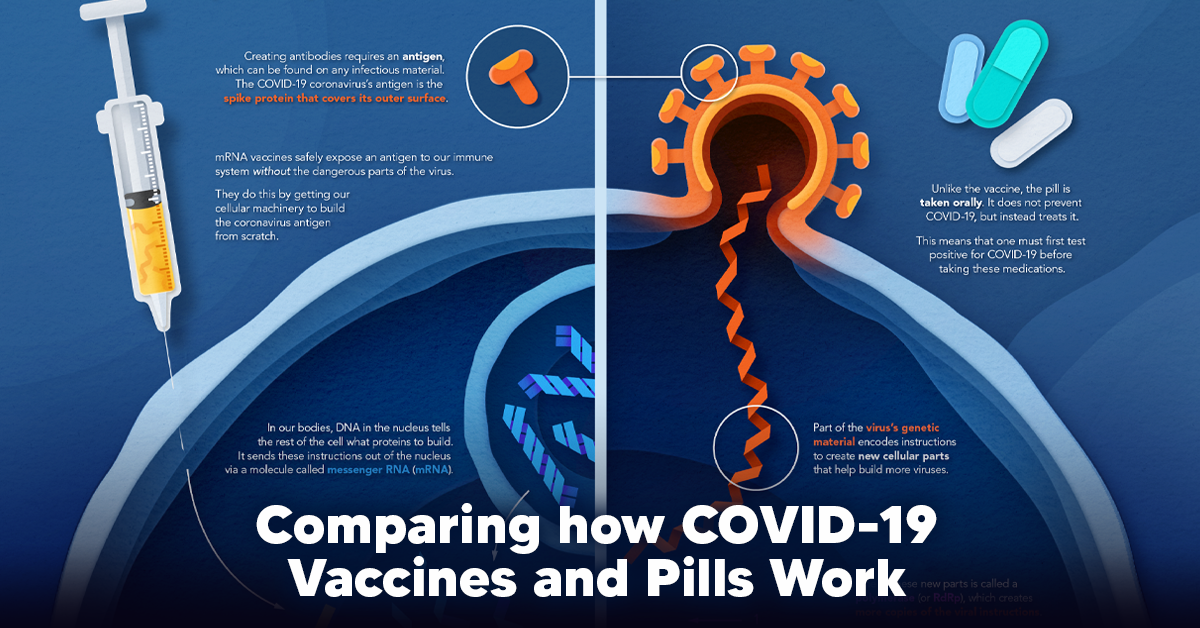
How a Vaccine Helps Prevent COVID-19
The main purpose of a vaccine is to prewarn the body of a potential COVID-19 infection by creating antibodies that target and destroy the coronavirus. In order to do this, the immune system needs an antigen. It’s difficult to do this risk-free since all antigens exist directly on a virus. Luckily, vaccines safely expose antigens to our immune systems without the dangerous parts of the virus. In the case of COVID-19, the coronavirus’s antigen is the spike protein that covers its outer surface. Vaccines inject antigen-building instructions* and use our own cellular machinery to build the coronavirus antigen from scratch. When exposed to the spike protein, the immune system begins to assemble antigen-specific antibodies. These antibodies wait for the opportunity to attack the real spike protein when a coronavirus enters the body. Since antibodies decrease over time, booster immunizations help to maintain a strong line of defense. *While different vaccine technologies exist, they all do a similar thing: introduce an antigen and build a stronger immune system.
How COVID Antiviral Pills Work
Antiviral pills, unlike vaccines, are not a preventative strategy. Instead, they treat an infected individual experiencing symptoms from the virus. Two drugs are now entering the market. Merck & Co.’s Lagevrio®, composed of one molecule, and Pfizer’s Paxlovid®, composed of two. These medications disrupt specific processes in the viral assembly line to choke the virus’s ability to replicate.
The Mechanism of Molnupiravir
RNA-dependent RNA Polymerase (RdRp) is a cellular component that works similar to a photocopying machine for the virus’s genetic instructions. An infected host cell is forced to produce RdRp, which starts generating more copies of the virus’s RNA. Molnupiravir, developed by Merck & Co., is a polymerase inhibitor. It inserts itself into the viral instructions that RdRp is copying, jumbling the contents. The RdRp then produces junk.
The Mechanism of Nirmatrelvir + Ritonavir
A replicating virus makes proteins necessary for its survival in a large, clumped mass called a polyprotein. A cellular component called a protease cuts a virus’s polyprotein into smaller, workable pieces. Pfizer’s antiviral medication is a protease inhibitor made of two pills: With a faulty polymerase or a large, unusable polyprotein, antiviral medications make it difficult for the coronavirus to replicate. If treated early enough, they can lessen the virus’s impact on the body.
The Future of COVID Antiviral Pills and Medications
Antiviral medications seem to have a bright future ahead of them. COVID-19 antivirals are based on early research done on coronaviruses from the 2002-04 SARS-CoV and the 2012 MERS-CoV outbreaks. Current breakthroughs in this technology may pave the way for better pharmaceuticals in the future. One half of Pfizer’s medication, ritonavir, currently treats many other viruses including HIV/AIDS. Gilead Science is currently developing oral derivatives of remdesivir, another polymerase inhibitor currently only offered to inpatients in the United States. More coronavirus antivirals are currently in the pipeline, offering a glimpse of control on the looming presence of COVID-19. Author’s Note: The medical information in this article is an information resource only, and is not to be used or relied on for any diagnostic or treatment purposes. Please talk to your doctor before undergoing any treatment for COVID-19. If you become sick and believe you may have symptoms of COVID-19, please follow the CDC guidelines.